Develop Better Products Faster
Modern SaaS solution allows medical device companies to track data required to successfully achieve key milestones.
Enlil
Enlil Inc.
Enlil Inc. forms digital threads through User Needs, Requirements, Test Cases, Standards as well as links to Software & Hardware versions, Engineering Builds and ultimately Lot History Records. All of this data forms the backbone to achieving milestones in the journey of a Device.
Solutions
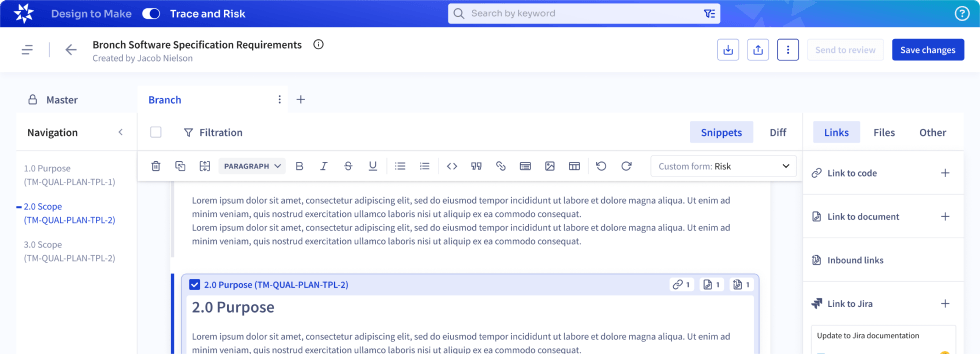
Trace and Risk

Our end-to-end solution traces design and test artifacts, streamlines decision-making, and brings products to market faster. Enlil offers customers guidance and an easy way to track and trace Requirements or Test Cases to important enterprise data such as Standards, Software code versioning systems, Engineering Builds, Quality Specs.
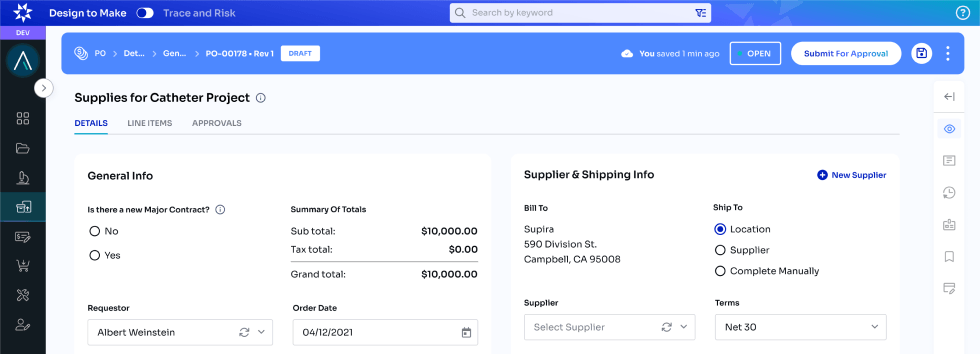
Design to Make
Enlil offers a Part 11 compliant cloud solution which allows users to track all crucial data during early product development. From auditing and approving documents, data and process to Parts, Equipment, Designs, Process, Enlil provides a seamless experience finding and linking all data needed to provide IP and get ready for Audits
Key features
Data to Insights
Make data-driven decisions and streamline innovation with Enlil’s powerful automation tools